According to Cook, the team is “planning to test our antibodies [against IL-11] in human safety trials by the end of 2020, and then start clinical trials with patients in 2021.”
Interleukin-11, a New Therapeutic Target in IPF
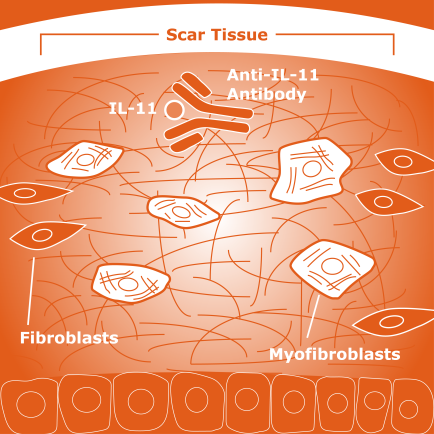
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease of unknown origin, characterized by lung tissue injury that is followed by the differentiation, or development of fibroblasts — connective tissue cells — into invasive myofibroblasts. Those myofibroblasts produce large amounts of proteins from the extracellular matrix (ECM) — the network that surrounds and supports cells — resulting in tissue scarring and loss of lung function.
Later studies then demonstrated that high levels of IL-11 led to tissue scarring in the heart and kidneys.
Taken together, these observations pointed toward the possibility that IL-11 might play a central role in tissue scarring in IPF.
To explore this possibility in more depth, the researchers from the MRC London Institute of Medical Sciences (LMS) and their collaborators performed a series of in vitro, or in the lab experiments using lung fibroblasts isolated from people with IPF.
The findings of the study, “Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis,” were published in the journal Science Translational Medicine.
As in their previous study, they found these fibroblasts produced high quantities of IL-11. However, the team also discovered that IL-11 led to the activation of genes that turned these lung fibroblasts into invasive myofibroblasts, which in turn produced large amounts of collagen and other ECM proteins.
The team forced mice lung fibroblasts to produce large quantities of IL-11 using genetic manipulation, or administered IL-11 directly to the animals. The lung fibroblasts differentiated — or became progressively more specialized — into myofibroblasts, and the animals ended up developing IPF.
However, when the scientists performed the same experiments in mice that had been genetically modified to lack a subunit of the IL-11 receptor, lung fibroblasts were unable to respond to the molecule and turn into myofibroblasts. For that reason, none of the animals developed the disease.
Results showed that the neutralizing antibody not only prevented lung fibroblasts from turning into invasive myofibroblasts. It also reduced inflammation and reversed existing tissue scarring in the animals’ lungs, suggesting that IL-11 could be explored as a future therapeutic target of IPF.
“We found that blocking the IL-11 protein with antibodies could reverse fibrosis in a mouse model of human lung disease. This is a remarkable finding as reversing fibrosis is tough to do. We believe this holds promise for treating fibrotic lung diseases, like IPF, in patients,” Stuart Cook, head of the cardiovascular disease mechanism group at the MRC LMS, and senior author of the study, said in a press release.
“This exciting research highlights the importance of IL-11 in driving the development of fibrosis, and gives hope for a new treatment approach to halt and maybe even reverse the devastating lung scarring of IPF,” said Toby Maher, a study co-author.
“Although we have treatments to slow disease progression, we desperately need new therapies to genuinely transform outcomes for people with IPF,” Maher said.
Adapted from pulmonaryfibrosisnews.com